In recent years, the world has paid more and more attention to rare earth elements, and many countries have listed them as key metal minerals. The U.S. Department of Energy calls them "technology metals". Therefore, rare earth metals have become strategic resources that the world's major economies compete for.

What are rare earth elements?
Rare earth elements (REEs) are a set of 17 metallic elements. These include the 15 lanthanides on the periodic table plus scandium and yttrium. According to the different properties and characteristics, the 17 rare earth elements are usually divided into 2 groups:
- Light rare earth elements: Lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, and gadolinium.
- Heavy rare earth elements: Terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, and yttrium.
Where can you find rare earth ores? You can find rare earth ores in China, Vietnam, and Brazil, etc.
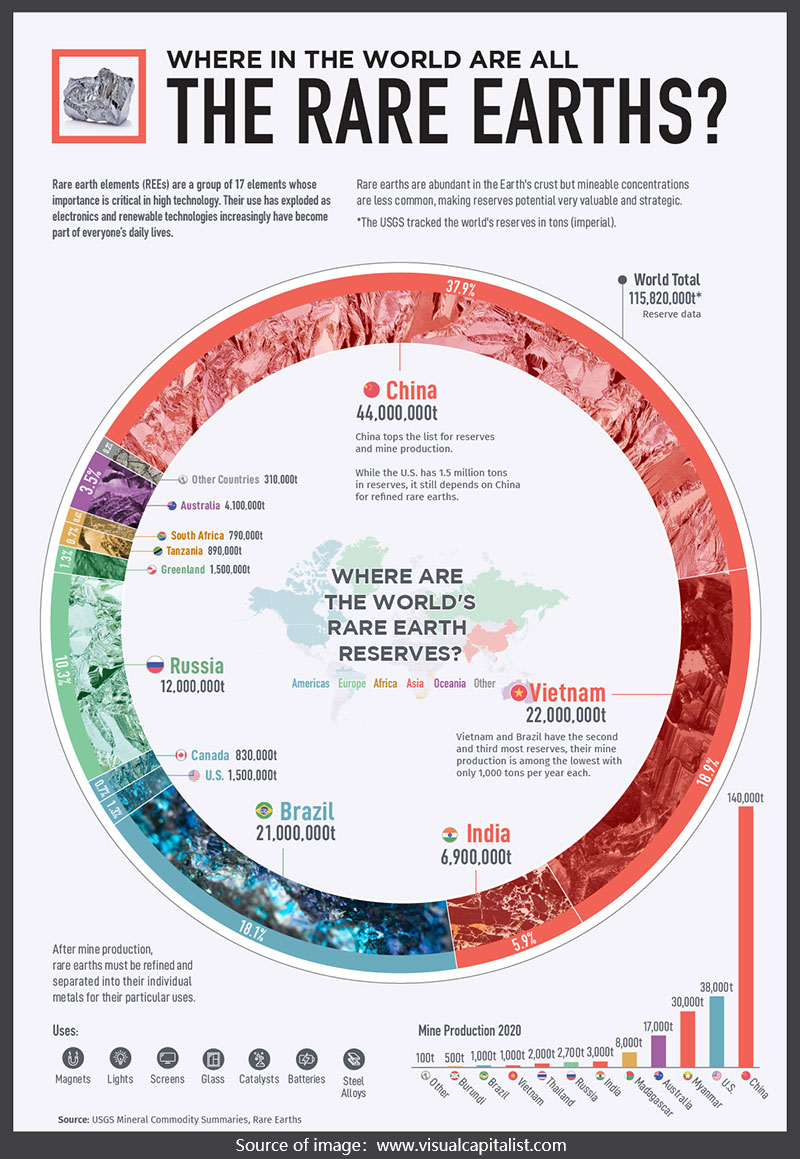
In the global reserves of rare earth mineral resources, China ranks first, Vietnam ranks second, and Brazil ranks third. Australia, Russia, the United States, and Canada are also rich in rare earth resources. In recent years, India, South Africa, Malaysia, Indonesia, and other countries have also discovered large rare earth deposits.
Next, we will explain in detail the properties and uses of the 17 rare earth elements.
1. Lanthanum (La)
Lanthanum is a soft and malleable silver-white metal and one of the most active rare earth elements. Scientists also call it "super calcium" because of its use in light conversion films. It is found in rare earth ores.
Physical properties of lanthanum

- Atomic number: 57
- Chemical symbol: La
- Density (g/cm³): 6.19
- Meltability (℃): 920±5
- Boiling point (℃): 4230
- Relative atomic weight: 138.9
What is lanthanum used for?
Metal lanthanum can produce nickel-metal hydride batteries and manufacture special optical glass. It is also used in various alloy materials such as piezoelectric materials, pyroelectric materials, magnetoresistance materials, luminescent materials, and laser materials.
2. Cerium (Ce)
Cerium is a silver-gray active metal, and its powder is easily oxidized in air and soluble in acid. Cerium is considered the most reactive element of all the rare earth metals except europium.
Physical properties of cerium

- Atomic number: 58
- Chemical symbol: Ce
- Density (g/cm³): 6.768
- Meltability (℃): 804±5
- Boiling point (℃): 2930
- Relative atomic weight: 140.1
What is cerium used for?
As a glass additive, cerium can absorb ultraviolet rays and infrared rays, widely used in automotive glass. The application of cerium in automobile exhaust catalysts can effectively reduce the emission of automobile exhaust gas. Cerium sulfide can color plastics and is also used in industries such as coatings, inks, and paper.
3. Praseodymium (Pr)
Praseodymium is a pale-yellow metal with a soft texture and ductility. Praseodymium and neodymium are two elements discovered at the same time. Praseodymium easily forms a green brittle oxide layer in the air.
Physical properties of praseodymium

- Atomic number: 59
- Chemical symbol: Pr
- Density (g/cm³): 6.769
- Meltability (℃): 935±5
- Boiling point (℃): 3020
- Relative atomic weight: 140.9
What is praseodymium used for?
Praseodymium is a large number of rare earth metals used in glass, ceramics, and magnetic materials. It makes orange-yellow stains for ceramics. Combinations with neodymium and praseodymium make high-powered magnets known for their strength and durability.
4. Neodymium (Nd)
Neodymium is a soft, silvery-white metal found primarily in monazite. It darkens rapidly in the air, forming oxides. Neodymium is associated with praseodymium, both of which are lanthanides with very similar properties.
Physical properties of neodymium

- Atomic number: 60
- Chemical symbol: Nd
- Density (g/cm³): 7.007
- Meltability (℃): 1024±5
- Boiling point (℃): 3180
- Relative atomic weight: 144.3
What is neodymium used for?
The biggest use of neodymium is Nd-Fe-B permanent magnetic material, which is the strongest known permanent magnet. It is known as the contemporary "King of Permanent Magnets", and is widely used in electronics, machinery, aerospace, and other industries with its excellent performance. Neodymium is also used in the coloring of glass, artificial rubies, and ceramic materials.
5. Promethium (Pm)
Promethium is the only natural radioactive rare earth element, known as the "artificial night pearl". In the dark, promethium glows pale blue or green due to its high radioactivity.
Physical properties of promethium

- Atomic number: 61
- Chemical symbol: Pm
- Density (g/cm³): 6.773
- Meltability (℃): 931±5
- Boiling point (℃): 3512
- Relative atomic weight: 140.908
What is promethium used for?
As a heat source, promethium provides auxiliary energy for vacuum detection and artificial satellites. Promethium is also widely used in steel manufacturing, plastics, and paper industry.
6. Samarium (Sm)
Samarium is a silvery metal with medium hardness and similar hardness and density to zinc metal. Samarium accounts for 2.8% of various minerals, including diatomite, yttrium niobate, monazite, and bastnaesite.
Physical properties of samarium

- Atomic number: 62
- Chemical symbol: Sm
- Density (g/cm³): 6.773
- Meltability (℃): 1025±5
- Boiling point (℃): 1630
- Relative atomic weight: 150.4
What is samarium used for?
Samarium is the raw material for making samarium-cobalt permanent magnets. Samarium cobalt magnets are the first rare earth magnets to be used in industry. Samarium is used in the manufacture of laser materials and infrared equipment. It also has important uses in the atomic energy industry.
7. Europium (Eu)
Europium is the least dense, softest, and most volatile metal element among the rare earth elements. Europium, mainly found in monazite and bastnaesite, is the rarest rare earth element.
Physical properties of europium

- Atomic number: 63
- Chemical symbol: Eu
- Density (g/cm³): 5.166
- Meltability (℃): 826±10
- Boiling point (℃): 1490
- Relative atomic weight: 152
What is europium used for?
As a light source, europium was used to produce visible light in fluorescent light bulbs, which helped popularize early color televisions. In recent years, europium oxide has been used in stimulated phosphors in new X-ray medical systems, in the manufacture of colored lenses and optical filters.
8. Gadolinium (Gd)
Gadolinium, silver-white metal, is regarded as the most special of the rare earth elements. Gadolinium is magnetic at room temperature, relatively stable in dry air, and tarnishes in humid air.
Physical properties of gadolinium

- Atomic number: 64
- Chemical symbol: Gd
- Density (g/cm³): 7.868
- Meltability (℃): 1350±20
- Boiling point (℃): 2730
- Relative atomic weight: 157.26
What is gadolinium used for?
Gadolinium is a noteworthy rare earth element in healthcare, which applies to the medical field. It shields nuclear reactors and neutron radiography. It improves magnetic resonance imaging (MRI), which aids in tumor treatment and the detection of tumor growth. In addition, magnetized refrigeration gadolinium can approach absolute zero.
9. Terbium (Tb)
Terbium is a silver-gray rare earth metal that is toxic. It is malleable and soft enough to be cut with a knife. Terbium is easily corroded by air at high temperature and corrodes very slowly at room temperature.
Physical properties of terbium

- Atomic number: 65
- Chemical symbol: Tb
- Density (g/cm³): 8.253
- Meltability (℃): 1336
- Boiling point (℃): 2530
- Relative atomic weight: 158.93
What is terbium used for?
Terbium applications mostly involve technology-intensive cutting-edge projects. For example, terbium can be used in activators, magneto-optical storage materials, magneto-optic glass, etc. In recent years, magneto-optical materials made of terbium have been mass-produced. They are used as computer storage components, and the storage capacity is increased by 10 ~ 15 times.
10. Dysprosium (Dy)
Dysprosium is a soft, silvery metal with a lively nature. Dysprosium has one of the highest magnetic strengths. It reacts rapidly with water and is soluble in acids.
Physical properties of dysprosium

- Atomic number: 66
- Chemical symbol: Dy
- Density (g/cm³): 8.565
- Meltability (℃): 1485±20
- Boiling point (℃): 2330
- Relative atomic weight: 162.51
What is dysprosium used for?
Dysprosium plays an important role in many high-tech fields. Dysprosium is used as an additive to rare earth permanent magnets to increase their efficiency at higher temperatures. Dysprosium can be used to prepare dysprosium lamps, which have the advantages of high brightness, good color, and small size. They have been used as lighting sources for movies and printing.
11. Holmium (Ho)
Holmium is similar to dysprosium in that it is soft and malleable. Holmium is stable in dry air and oxidizes quickly at high temperatures. Holmium oxide is the most paramagnetic substance known.
Physical properties of holmium

- Atomic number: 67
- Chemical symbol: Ho
- Density (g/cm³): 8.799
- Meltability (℃): 1490
- Boiling point (℃): 2330
- Relative atomic weight: 164.94
What is holmium used for?
Holmium alloys create flux concentrators that produce the strongest magnetic fields. Holmium can be used as an additive for metal halide lamps, yttrium iron, and yttrium aluminum garnet. It is also widely used in the production of optical communication devices.
12. Erbium (Er)
Erbium is a silver-gray metal and has outstanding optical properties. It is an important rare earth element in the nuclear region. Erbium is strongly ferromagnetic near absolute zero and is a superconductor.
Physical properties of erbium

- Atomic number: 68
- Chemical symbol: Er
- Density (g/cm³): 9.058
- Meltability (℃): 1500-1550
- Boiling point (℃): 2630
- Relative atomic weight: 167.27
What is erbium used for?
Erbium oxide (Er₂O₃) is rose red and is used to make pottery glaze. Erbium is used in the military for laser rangefinders, and can also be used as an activator for certain fluorescent materials. In addition, erbium can be used in the decolorization and coloring of spectacle lens glass and crystal glass.
13. Thulium (Tm)
Thulium is the second rarest metal in the lanthanide series, after promethium. Thulium has a bright silver-gray luster and is relatively stable in the air. Thulium oxide is a light green crystal.
Physical properties of thulium

- Atomic number: 69
- Chemical symbol: Tm
- Density (g/cm³): 9.318
- Meltability (℃): 1500-1600
- Boiling point (℃): 2130
- Relative atomic weight: 168.94
What is thulium used for?
Thulium is used in the clinical diagnosis and treatment of tumors because of its high affinity for tumor tissues. Thulium is used as an additive in new light sources (metal halide lamps) and is also used as a light source for medical portable X-ray machines.
14. Ytterbium (Yb)
Ytterbium is a silvery-white soft metal that is shiny and easily oxidized. The oxide is white. Ytterbium is mainly found in xenotime and euxenite.
Physical properties of ytterbium

- Atomic number: 70
- Chemical symbol: Yb
- Density (g/cm³): 6.959
- Meltability (℃): 824±5
- Boiling point (℃): 1530
- Relative atomic weight: 173.04
What is ytterbium used for?
Due to its excellent spectral properties, ytterbium is used as a high-quality laser material. It can be used to make laser glass and fiber lasers. Ytterbium is also used in shielding coating materials, pressure sensor materials, magnetostrictive materials, and high-tech alloy additive materials.
15. Lutetium (Lu)
As the last of the lanthanides, lutetium is the hardest and densest metal among the rare earth elements. Lutetium is relatively stable in the air. Lutetium oxide is a colorless crystal.
Physical properties of lutetium

- Atomic number: 71
- Chemical symbol: Lu
- Density (g/cm³): 9.849
- Meltability (℃): 1650-1750
- Boiling point (℃): 1930
- Relative atomic weight: 174.99
What is lutetium used for?
Lutetium can be used in energy battery technology. Stable lutetium nuclides can be used as catalysts for petroleum cracking, alkylation, hydrogenation, and polymerization. Additionally, lutetium has several interesting uses. For instance, lutetium isotopes can help reveal the age of ancient objects.
16. Scandium (Sc)
Scandium, a silvery-white metal, is one of two non-lanthanide rare earth elements. Scandium mainly exists in very rare scandium yttrium stone, which is rapidly oxidized in the air and loses its luster.
Physical properties of scandium

- Atomic number: 21
- Chemical symbol: Sc
- Density (g/cm³): 2.995
- Meltability (℃): 1550-1600
- Boiling point (℃): 2750
- Relative atomic weight: 44.97
What is scandium used for?
In the electronics industry, scandium is often used in a variety of semiconductor devices. In the metallurgical industry, scandium is used to make alloys to improve their strength, hardness, and heat resistance. In the glass industry, special glass containing scandium can be manufactured. In the electric light source industry, scandium sodium lamps have the advantages of high efficiency and positive light color.
17. Yttrium (Y)
Yttrium, a gray-black metal, is another non-lanthanide rare earth element. In the earth's crust, yttrium and cerium are the two most abundant rare earth elements, and they were also the first rare earth elements discovered.
Physical properties of yttrium

- Atomic number: 39
- Chemical symbol: Y
- Density (g/cm³): 4.472
- Meltability (℃): 1552
- Boiling point (℃): 3030
- Relative atomic weight: 88.92
What is yttrium used for?
Yttrium has many uses in industry. As an additive to steel and non-ferrous alloys, FeCr alloys usually contain 0.5-4% yttrium, which can enhance the oxidation resistance and ductility of stainless steel. Silicon nitride ceramic materials containing 6% yttrium and 2% aluminum can be used to develop engine components.
References
1. Rare Earth Elements Facts
2. Rare Earth Elements and Their Uses
3. Rare Earth Elements: Where in the World Are They?